Non-metallic batteries
The Murray group works on designing transition metal-free systems for energy storage and conversion. This includes small-molecule mediators and design of conjugated and non-conjugated polymers for flow and solid-state batteries. We also design electrochemical systems for environmental remediation and sensing. The group consists of a mixture of organic synthesis and electrochemical techniques Our aim is to design low-cost, electronically tunable systems that are amenable to the production of energy storage devices.
Tunable organic flow batteries
Energy storage is quickly becoming one of the most pressing issues for the implementation of a renewable energy economy and thus a world with standards of living decoupled from CO2 emissions. One of many proposed solutions is the redox flow battery (RFB). These are essentially rechargeable fuel cells, and can be scaled to almost any size. Limitations, however, on current systems based on vanadium, are the expense and limited supply of vanadium. Ideally, metal-free battery materials with rapid and reversible kinetics would be able to replace vanadium in redox-flow batteries. Current promising examples exist but can suffer from high toxicity or limited solubility.
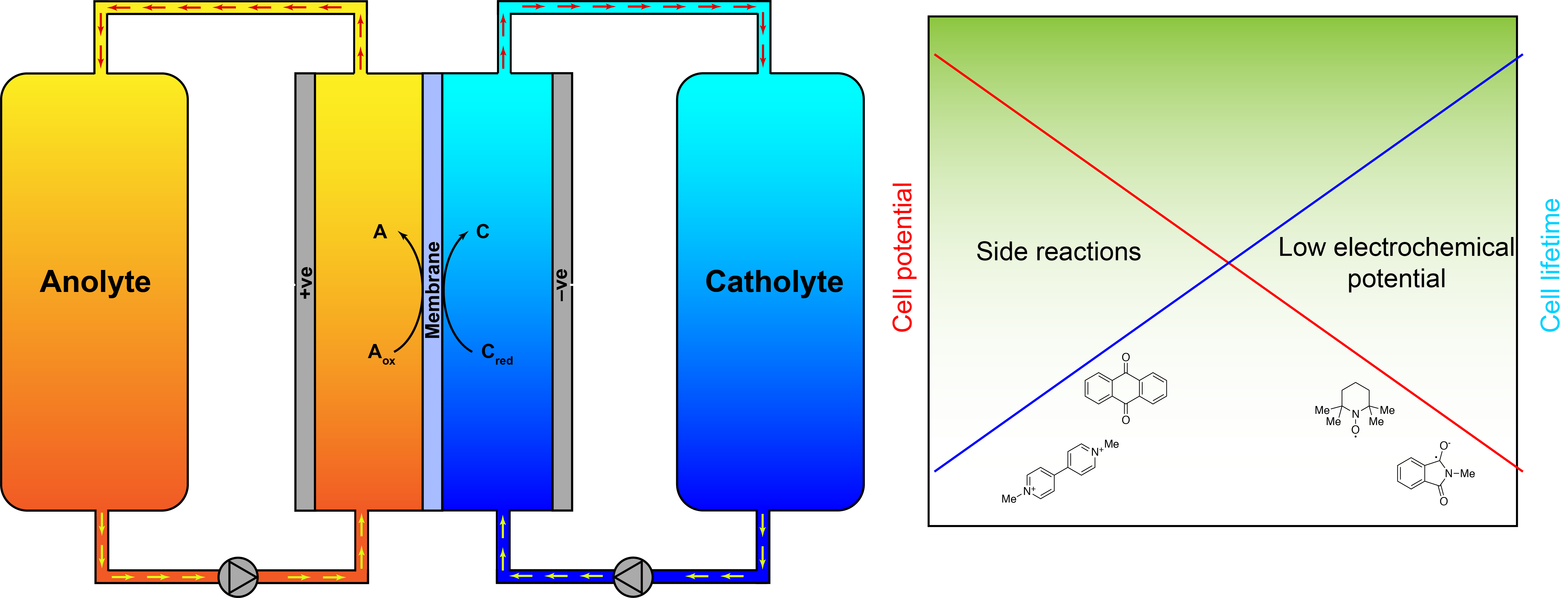
Furthermore, there is an inherent difficulty in finding reactive intermediates with rapid redox kinetics and high cell potential, but low propensity to undergo side reactions. This can be rectified by electronic tuning of conjugated organic mediators. Electronically conjugated nitroxyl radicals, as well as NADH-type analogues, are potential solutions to these problems.
Metal-free energy storage materials
Conjugated redox-active polymers are crucial for energy storage both in the traditional field of ion intercalation-based batteries and of so called "supercapacitors" which generally rely on Faradaic pseudocapacitive materials to store and transduce energy. We will design a series of metal-free materials which can intercalate traditionally challenging substrates such as sodium ions.
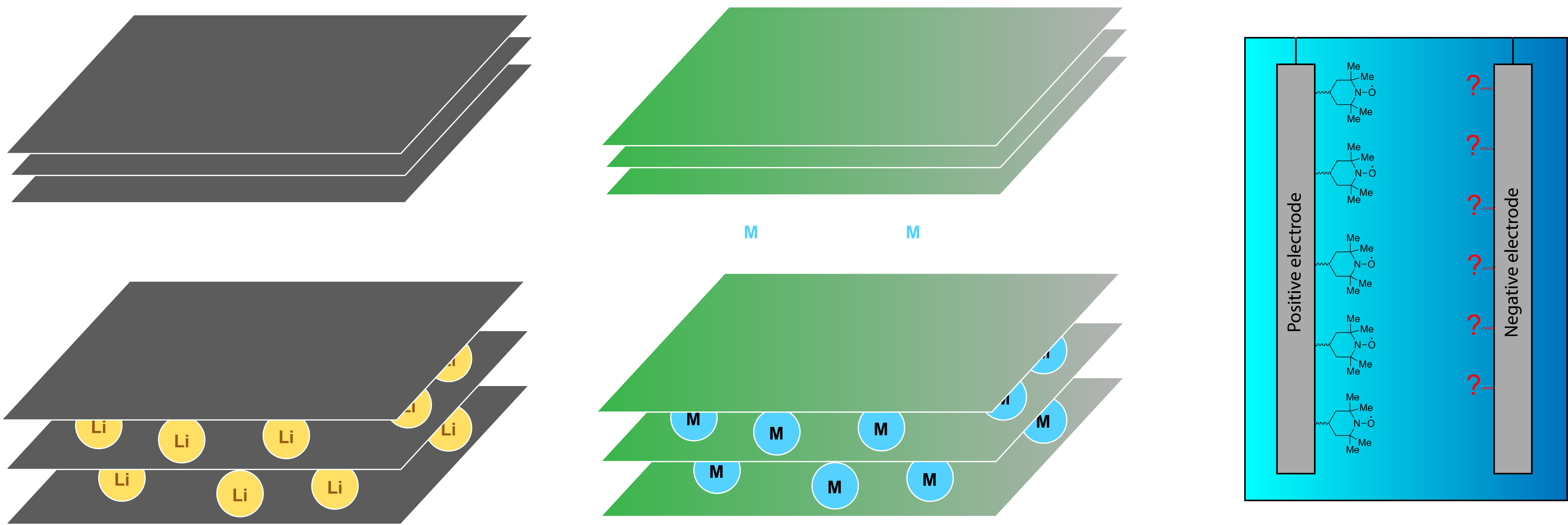
Additionally, non-conjugated ('organic radical') polymers bearing redox active discrete moieties have attracted interest as solid-state battery materials. However, often they have been used only as cathode materials with a Li metal anode. We will design negative terminal electrodes based on organic redox-active polymer units attached to an inert backbone for the generation of all-organic solid state batteries.